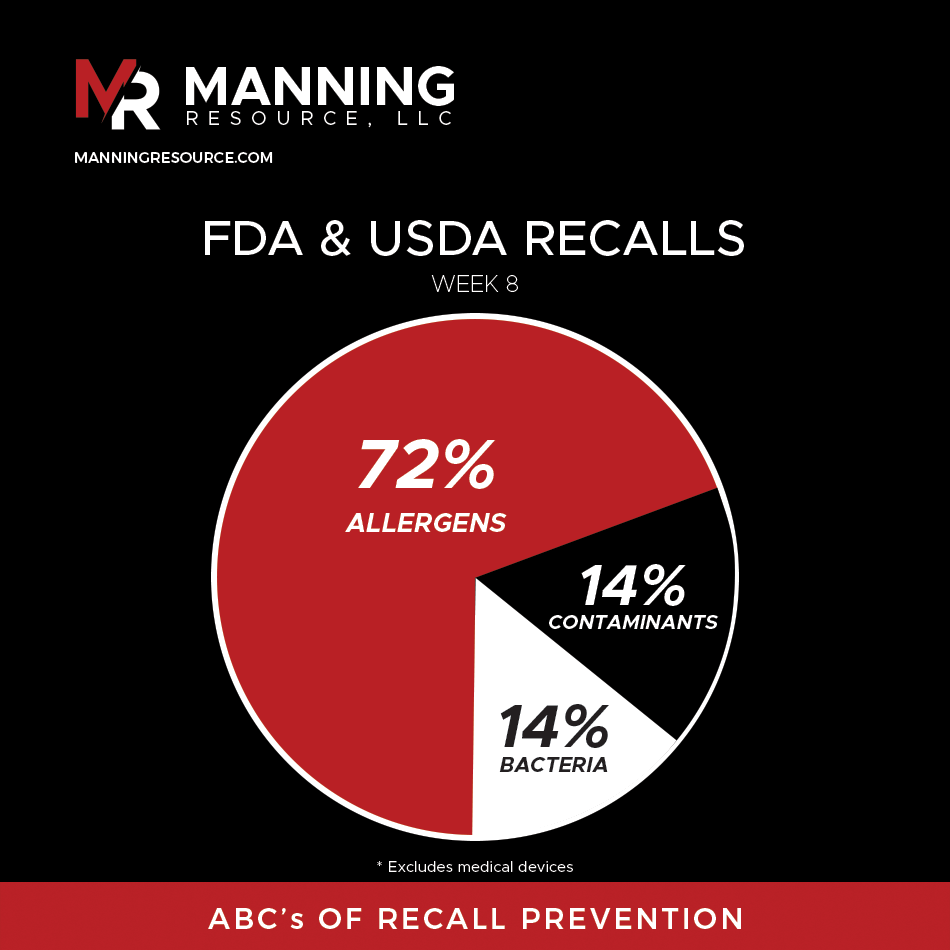
Week 8 Recall Overview (Feb 16 – Feb 23, 2025)
Seven recalls were issued in the food and beverage sector, six by the FDA and one by USDA. Issues ranged from potential Listeria contamination in frozen supplemental shakes to several cases of undeclared allergens and misbranding in various products.
Key Incidents:
- Frozen Supplemental Shakes (2/23): Possible Listeria contamination led to an immediate halt in distribution to protect long-term care facility consumers.
- Dark Chocolate Conettos (2/21): A labeling error failed to declare milk allergens.
- Snack Rolls, Biscuits, and Wafers (2/20): A consumer complaint uncovered missing allergen information (wheat, eggs, milk) due to supplier miscommunication.
- Vitality Capsules (2/20): Dietary supplements were recalled after tests found undeclared sildenafil and tadalafil.
- Botana Mix Snacks (2/20): Mislabeling resulted in undeclared allergens including wheat, sesame, soy, and certain color additives.
- Berry Buddies Bento Box (2/19): A production error led to an incorrect label that omitted wheat and eggs allergens.
- Chicken Caesar Wrap (USDA, 2/23): Routine quality checks revealed misbranding and an undeclared fish allergen.
Recall Prevention Focus:
- Supplier Oversight: Regular audits and clear communication on ingredient disclosures are critical.
- Stringent Quality Assurance: Routine checks and robust verification processes help catch errors before products hit the market.
- Effective Production Protocols: Standardizing changeover procedures and ensuring accurate multilingual labeling can prevent mislabeling.
- Quick Response: Immediate action upon detecting issues—like halting purchases and notifying customers—minimizes risks.
- Employee Training: Ongoing training on quality assurance and allergen management helps keep standards high.
Taking these steps can significantly reduce the risk of future recalls and ensure consumer safety and brand integrity.



Add Comment