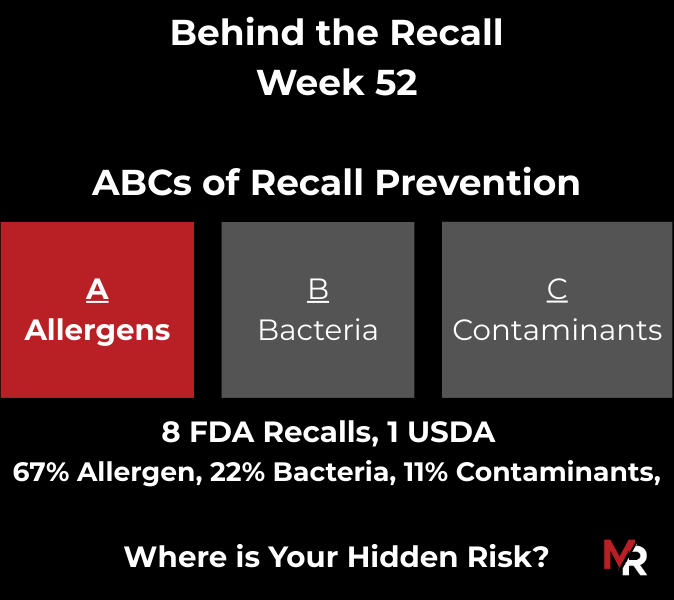
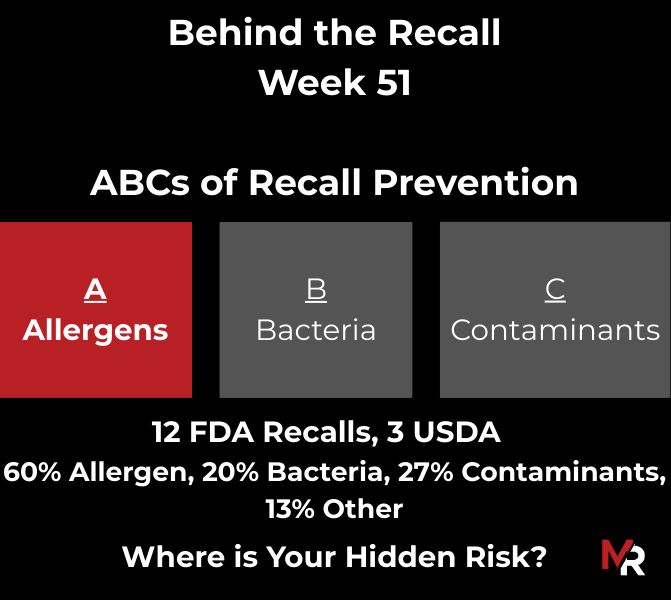
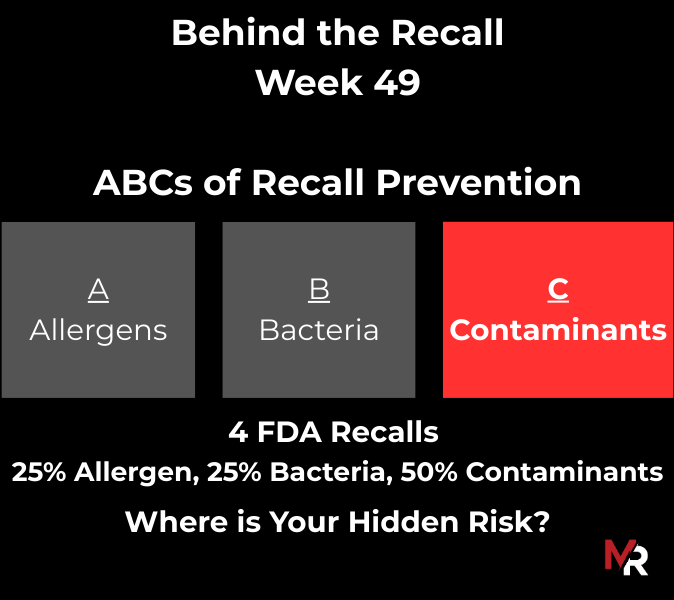
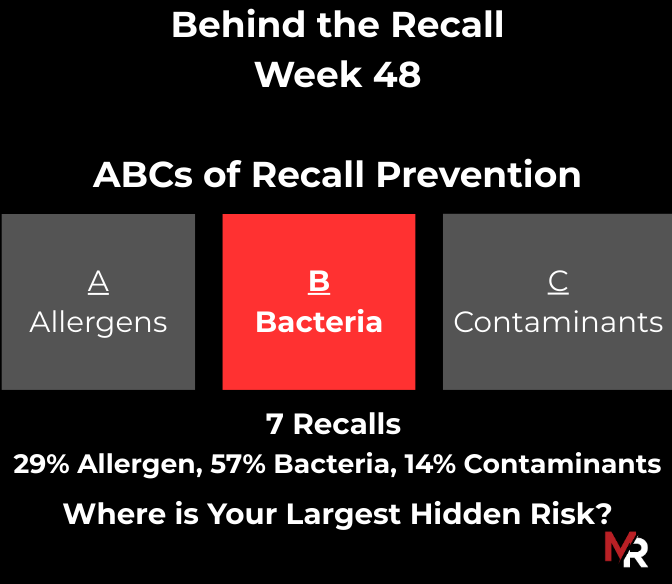
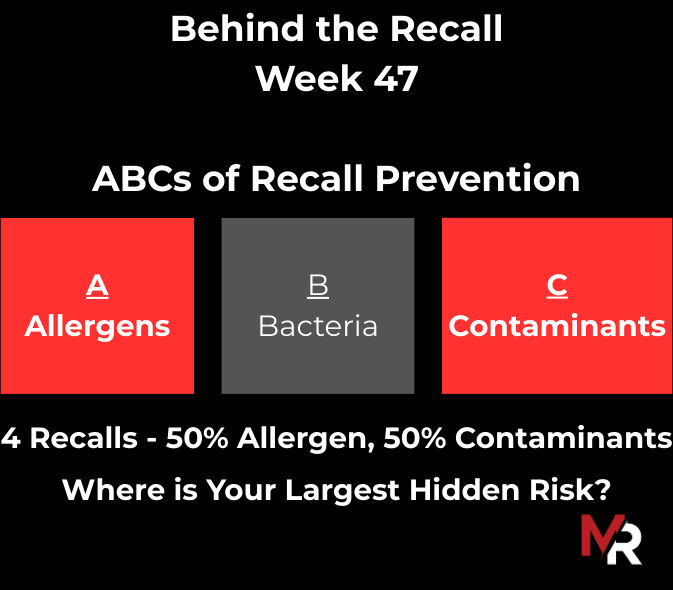
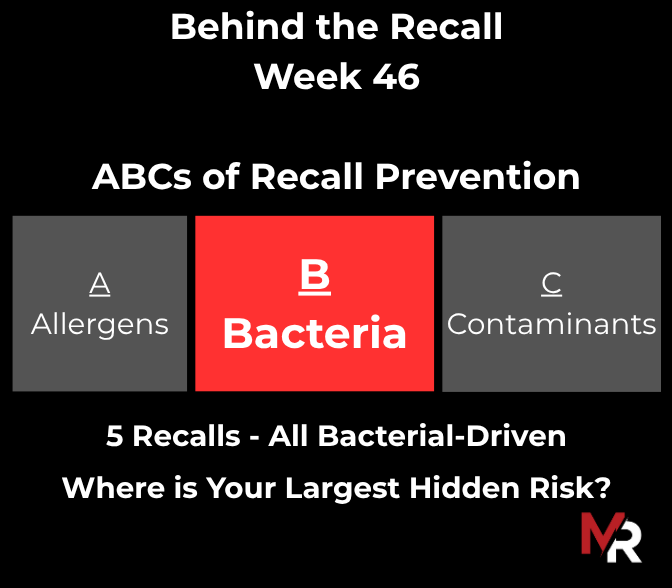
Behind the Recall – Week 52 This week’s recalls weren’t driven by exotic hazards. They came from everyday breakdowns, storage conditions, labeling execution, sanitation discipline, and pathogen findings that were
Current Class 1 FDA & USDA Recall Trends FDA Recalls / Reason(s) 12/20/25 – Food & Beverage – Almondmilk Chocolate 46% Madagascar Plant-Based – Contains undeclared hazelnut. “We have received
Current Class 1 FDA & USDA Recall Trends – No USDA Recalls Listed FDA Recalls / Reason(s) 12/5/25 – Food & Beverage – Deluxe Mixed Nuts Unsalted (34 oz tubs
Current Class 1 FDA & USDA Recall Trends FDA Recalls / Reason(s) 11/26/25 – Food & Beverage – Grated Pecorino Romano Cheese – Potential presence of Listeria monocytogenes. “The recall
Current Class 1 FDA & USDA Recall Trends FDA Recalls / Reason(s) 11/20/25 – Food & Beverage – Lava Buns – Undeclared milk allergen. “The issue was identified during an
Current Class 1 FDA & USDA Recall Trends FDA Recalls / Reason(s) 11/15/25 – Food & Beverage – Vampire Slayer Garlic Cheddar cheese – Potential to be contaminated with Listeria
November 2, 2025 – November 8, 2025 – 13 Recalls (12 FDA, 1 USDA) Current Class 1 FDA & USDA Recall Trends FDA Recalls / Reason(s) 11/8/25 – Food &
Current Class 1 FDA & USDA Recall Trends FDA Recalls / Reason(s) 10/31/25 – Food & Beverage – Bali Gold, Red Bali, Green Maeng Da, and White Elephant Kratom powder
Current Class 1 FDA & USDA Recall Trends FDA Recalls / Reason(s) 10/4/25 – Food & Beverage – Basil Pesto Bowtie Salad and Smoked Mozzarella Penne Salad – Potential Foodborne
Current Class 1 FDA & USDA Recall Trends FDA Recalls / Reason(s) 9/27/25 – Food & Beverage – Dried Bean Curd – Undeclared wheat allergen. “The recall was initiated after