Industries/Products: Confections, Fruit, Eggs, Prepared Foods, Medical Devices
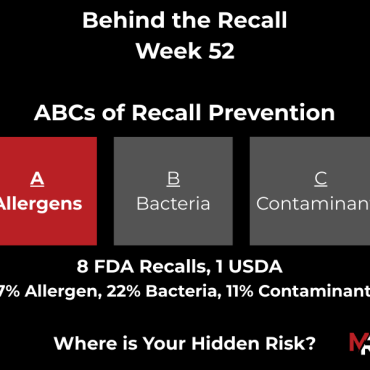
Current FDA & USDA Recall Trends
FDA
Recalls / Contributing Factor(s)
- 9/6/24 – Food & Beverage – Dark Chocolate Filled Mini Waffle Cones – Undeclared Milk.
- “The recall was initiated after the firm received one consumer report of an allergic reaction experienced after consuming the product. As part of the firm’s ongoing investigation, the product was tested and found to contain more than trace amounts of milk proteins, which is not indicated on the packaging. The firm’s investigation into the cause is ongoing.” 1
- 9/6/24 – Food & Beverage – Whole Cantaloupe – Potential to be contaminated with Salmonella.
- “The recall is the result of routine sample testing conducted by the State of Michigan which reveled the presence of Salmonella in cantaloupe sold at retail.” 2
- 9/6/24 – Food & Beverage – Eggs – Potential to be contaminated with Salmonella.
- “The recall was initiated after the FDA informed the company that environmental samples tested positive for the bacteria. FDA also conducted whole genome sequencing and found that the samples were related to an ongoing Salmonella outbreak investigation.” 3
- 9/5/24 – Food & Beverage – Shiso Katsuo Ninniku & Miso Katsuo Ninniku – Undeclared Fish (Bonito).
- “The recall was initiated after it was discovered that product containing Fish (Bonito) was distributed in packaging that did not reveal the presence of “Containing allergen: Fish (Bonito)”.” 4
- 9/4/24 – Medical Devices – Bivona® Aire-Cuf®, TTS™, Uncuffed, Mid-Range Neonatal/Pediatric Tracheostomy Tube(s) and Bivona Aire-Cuf®, TTS™, Cuffless FlexTend™, TTS™ FlexTend™ Adult Tracheostomy Tube(s) – The securement flange of specific lots of the Bivona Neonatal/Pediatric and Adult Tracheostomy products may tear because of a manufacturing defect.
- “The customer notification sent on May 29, 2024, indicated that if the flange on the item is torn or broken, the tracheostomy tube may not stay in position in the trachea. This can lead to tracheostomy displacement or decannulation. Either event may result in an inability to properly ventilate or protect the airway and may contribute to a catastrophic adverse event.” 5


Add Comment