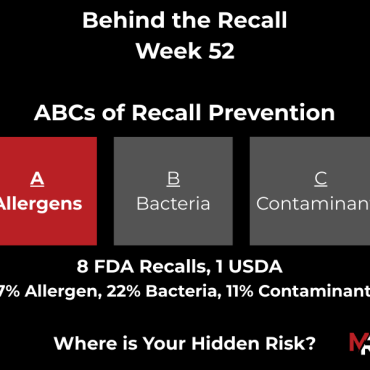
November 2, 2025 – November 8, 2025 – 13 Recalls (12 FDA, 1 USDA)
Current Class 1 FDA & USDA Recall Trends
FDA
Recalls / Reason(s)
- 11/8/25 – Food & Beverage – Whole Nutrition Infant formula – Potential Foodborne Illness – Clostridium botulinum.
- “ByHeart was notified by the FDA on November 7, 2025 of an estimated 83 cases of infant botulism that were reported nationwide since August 2025. Of these, the FDA also noted that 13 infants received ByHeart formula at some point. The FDA has not identified a direct link between any infant formula and these cases and there is no historical precedent of infant formula causing infant botulism. Botulism is extremely uncommon in dairy products or infant formula, and is naturally occurring in environmental sources like soil, select vegetables, and dust.” 1
- 11/7/25 – Drugs – Famotidine Injection, USP 20 mg per 2 mL – Out-of-specification endotoxin results.
- “The product is being recalled due to out-of-specification (OOS) endotoxin results of certain reserve samples from a single lot. Based upon the investigation, two additional lots were also included in the recall as a precautionary measure.” 2
- 11/6/25 – Food & Beverage – English muffin – Undeclared milk.
- “Cincinnati, Ohio (October 31, 2025) Blue Oven Bakery, Inc. is issuing a voluntary recall on their english muffin for misbranding due to the milk allergen not being declared on the label. The english muffin was sold fresh as a baked product in a 13.04 oz sealed plastic bag.” 3
- 11/6/25 – Food & Beverage – Oven Dried Fish (Scomberomorus cavalla) – Potential to be contaminated with Clostridium botulinum.
- “African Food on Wheels Inc. of Bronx, NY is recalling 28 boxes of Product, because it is a dried, un-eviscerated (internal organs not removed) fish greater than 5 inches and length and has the potential to be contaminated with Clostridium botulinum, a bacterium which can cause life-threatening illness or death. Consumers are warned not to use the product even if it does not look or smell spoiled.” 4
- 11/6/25 – Food & Beverage – Organic Moringa Leaf Powder – Potential to be contaminated with Salmonella.
- “Africa Imports is voluntarily recalling its Organic Moringa Leaf Powder (1 kilogram box) because it may be contaminated with Salmonella, an organism that can cause serious and sometimes fatal infections in young children, the elderly, and individuals with weakened immune systems. Healthy persons infected with Salmonella may experience fever, diarrhea (which may be bloody), nausea, vomiting, and abdominal pain. In rare cases, infection can lead to more severe conditions such as arterial infections, endocarditis, or arthritis.” 5
- 11/4/25 – Dietary Supplements – Tejocote Root dietary supplement – Product contains yellow oleander.
- “SiluetaYa is recalling its tejocote root because FDA analysis has determined that the products appear to be Thevetia peruviana, or yellow oleander. All parts of the yellow oleander plant are known to contain cardiac glycosides, which are highly toxic to humans and animals. Ingestion of yellow oleander can cause severe or even fatal neurological, gastrointestinal, and cardiovascular adverse health effects. Symptoms may include nausea, vomiting, dizziness, diarrhea, abdominal pain, changes in heart rhythm, arrhythmia, and more.” 6
- 11/4/25 – Food & Beverage – Peach Salsa – Potential to be contaminated with Listeria monocytogenes.
- “This action is in response to the October 29, 2025, recall issued by Moonlight Companies, whose peaches were supplied to retailer partners, used as ingredients in our Peach Salsa and sold through Kroger.” 7
- 11/4/25 – Food & Beverage – Ice Cream Bars – May contain undeclared wheat.
- “No illnesses or injuries have been reported to date. We are recalling this product because it may contain products that contain wheat in packaging that does not reveal the presence of wheat on the label. Although our investigation is ongoing, we believe products containing wheat were repacked into the incorrect packaging at the beginning of a production run.” 8
- 11/3/25 – Drugs – 20 mEq Potassium Chloride Injection – Potential for Potassium chloride overdose: 20 mEq Potassium Chloride Injection is Mislabeled As 10 mEq Potassium Chloride Injection.
- “FOR IMMEDIATE RELEASE – October 31, 2025 AUSTIN, TX – Otsuka ICU Medical LLC is issuing a voluntary recall to the user level, for a MISLABELLED lot of POTASSIUM CHLORIDE Inj. 20 mEq, NDC 0990-7077-14. The OVERWRAP label of lot 1030613, Expiration Date: 09-30-2026 may incorrectly identify the product as POTASSIUM CHLORIDE Inj. 10 mEq with NDC 0990-7074-26. Otsuka ICU Medical LLC has identified this discrepancy due to a manufacturing issue. The dosage is correctly printed on the labeling affixed to the product bag which is not visible when the 10 mEq OVERWRAP is in place. This notification details the issue and the required steps for you to perform.” 9
- 11/3/25 – Medical Devices – Bronchofiberscopes and bronchovideoscopes – Potential for endobronchial combustion.
- “There is a risk of endobronchial combustion if laser therapy, argon plasma coagulation, or high-frequency cauterization is performed while supplying oxygen in excess of 40% and/or the electrode section of the electrosurgical accessory is too close to the distal end of the endoscope.” 10
- 11/3/25 – Food & Beverage – Peach Salsa – Potential to be contaminated with Listeria monocytogenes.
- “The product is being recalled due to peaches purchased from our retailer have the potential to be contaminated with Listeria monocytogenes, an organism which can cause serious and sometimes fatal infections in young children, frail or older adults, and others with weakened immune systems. Although healthy individuals may suffer only short-term symptoms such as high fever, severe headache, stiffness, nausea, abdominal pain and diarrhea, Listeria infection can cause miscarriages and stillbirths among pregnant women.” 11
- 11/3/25 – Food & Beverage – Marinara Sauce, Puttanesca Sauce, and Meat Flavored Pasta Sauce – Potential for Clostridium botulinum hazard as the product is manufactured without an approved schedule.
- “The products were manufactured without an approved scheduled process or otherwise evaluated to determine if the process is adequate. Failure to appropriately process acidified or low-acid canned foods can result in Clostridium botulinum toxin formation.” 12
USDA
- 11/4/25 – Food – Frozen Chicken and Beef Croquette Products – Misbranding and an Undeclared Allergen – The problem was discovered when the Food and Drug Administration notified FSIS that bread crumb ingredients shared between FDA-regulated and FSIS-regulated products produced at the facility contained sesame that may not have been declared on the labels of the FSIS-regulated products.” 13


Add Comment